Galvanizing myths and facts
Galvanizing is a widely used method for protecting steel from corrosion, and with its popularity comes a number of myths and misconceptions. We outline some of the most common galvanizing myths, and share the facts about this steel coating method.

Myth: Galvanizing is a new practice
Fact: Galvanizing was first developed in the early 19th century; the first patent for coating steel in molten zinc was formed in 1836. It has since become a cornerstone of corrosion protection technology. The longevity of galvanized structures around the world, many still in service after more than a century, demonstrates the proven reliability and effectiveness of the process. The galvanizing process continues to evolve with new innovations and applications.
Joseph Ash Galvanizing has been around nearly as long as hot dip galvanizing, forming all the way back in 1857.
Myth: Galvanizing is only for large structures
Fact: While galvanizing is commonly associated with large infrastructure like structural steel, it is equally effective for smaller components, such as nuts, bolts, fasteners and fixings.
However, the process needs to be adapted slightly to make it suitable for small steel pieces. A coating process known as spin galvanizing involves placing the steel into a basket first, before being centrifuged – or spun – at high speeds to remove the excess zinc.
Myth: Galvanized steel can’t be painted
Fact: Paint can be applied to galvanized steel, adding an extra layer of protection and enhancing its visuals. However, it does require the correct surface preparation. The smooth, shiny finish of freshly galvanized steel can resist paint adhesion, so proper surface treatment and the correct type of paint are needed to maxiumum effectiveness.
Galvanized steel is also suitable for powder coating, an alternative to paint that lasts longer. Galvanizing and powder coating used on the same fabrication is known as a duplex coating, providing an extended lifespan while leaving an aesthetically pleasing finish.
Myth: Galvanized steel can’t be recycled
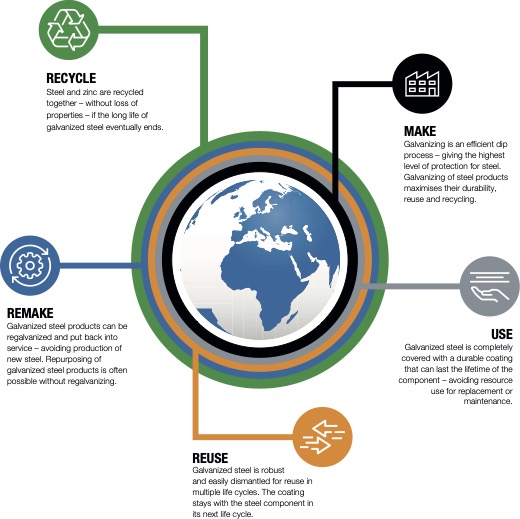
Fact: Galvanized steel is fully recyclable, without loss of properties. Both the hot dip galvanizing process and the galvanized product itself fit into the principles of a circular economy.
Plants at Joseph Ash Galvanizing have also been adapted to capture as many emissions as possible, reducing environmental impact. Our interactive animation shows exactly how we keep the process sustainable at every stage of the process.
Myth: Galvanized steel is more expensive in the long term
Fact: While initial cost of galvanizing can be higher than other coatings such as paint, it’s important to consider the long-term savings. Galvanized steel often requires little to no maintenance over the multiple decades it can last for, reducing the total lifetime cost of the upkeep. Additionally, its long life means fewer replacements, repairs, and disruptions, further contributing to cost savings.
The Galvanizer’s Association suggests that over a 25-year period, paint will cost 70% more than galvanizing. So even though it may seem more expensive at first glance, it is actually the cheaper option in the long term.
Are you looking for alternatives to paint for your steel fabrications? You may have heard about powder coating, but not be sure what it is or what it involves. In this blog, we cover everything there is to know about powder coating and how it is superior to traditional liquid paint.

What is powder coating?
Powder coating is a longer lasting coating alternative to traditional liquid paint. Instead of being applied in liquid form with a paintbrush or roller, it is applied in a powder format with an electrostatic spray gun.
The gun charges the powder particles with electrostatic charge, helping the particles to adhere to the metal surface. This process means that the finish achieved is much more durable and corrosion-resistant than traditional paint. Although frequently done on steel fabrications, it is also possible to powder coat aluminium.
When applied in combination with hot dip galvanizing, it helps protect steel against corrosion for multiple decades.
Can you powder coat aluminium?
Yes. Like steel, aluminium can be powder coated. However, unlike steel, aluminium cannot be hot dip galvanized.
How is powder coating performed?
Before coating, the steel’s surface must be thoroughly cleaned to remove any dirt, grease, or rust. The powder coating is then sprayed on using an electrostatic spray gun in the fabricator’s or specifier’s colour of choice. The powder itself is made up of finely ground particles of pigment and resin.
After application, the coated item is heated in an oven at high temperatures, typically between 175-200°C. The heat causes the powder to melt and flow, creating a smooth, even coating. The coating then solidifies and chemically reacts to form a hard, durable finish. This curing process provides low maintenance and long lasting corrosion protection for steel.

Why powder coating is better than paint
Powder coating offers several benefits over traditional paint.
Improved durability and corrosion resistance
Powder coating is more durable than traditional paint. It is resistant to chipping, scratching, and fading, leaving the fabrication looking nicer for longer. It also makes it cheaper over its lifetime due to a reduced need to maintain the coating.
Powder coating is also much more resistant to corrosion compared to liquid paint. This helps the underlying steel to last longer, too, reducing the need for complete refabrication.
Quicker drying time
Paint needs several days to dry after it has been applied. It also needs multiple coats to achieve the desired result. In contrast, a powder coat can be completed in one sitting and has a quicker drying time. This means you get your fabrication back quicker and spend less time waiting – ideal if you have a tight deadline you need to work towards.
Environmentally friendly
Powder coating is an environmentally friendly alternative to traditional paint. It produces less waste, and the powder can be recycled. Additionally, paint contains toxic solvents that damage the environment. The powder coating solution does not contain toxic solvents and is safer for operators to apply, provided the correct PPE is used for the job.
Lower lifetime costs
Powder coating is a cost-effective alternative to traditional paint, thanks to its increased durability and recyclability. The time and money that would otherwise be spent re-painting the steel on a regular basis is reduced, because the powder coating layer is much more durable.
What colours can I powder coat my steel?
If you can think of a colour, it is almost certainly available in powder coating. Powder coating paints come in many different colours, and can be used to create different finishes such as textured or metallic.
Powder coating options at Joseph Ash Medway can be categorised into one of five finishes:
- Anodic – anodised alternatives
- Futura – special effect, two-toned colours, textured metallics
- Structura – fine texture RAL shades
- Brilliance – bright sparkling metallics
- Traditional RAL colours

Anodic
Anodic is a range of exclusive metallic effect powder coating finishes – providing a great alternative to anodized aluminium. This range is supplied in Interpon D2525 ultra-durable polyester technology.
Futura
Futura is a range of special powder coating finishes for use on exterior architectural metal components.
Structura
Structura is a range of special powder coating finishes for use on exterior architectural metal components.
Brilliance
Brilliance is a range of revolutionary single-coat powder application that achieves a finish previously only realised using liquid coatings. The Brilliance finishes are available in different durability levels, which provide excellent interior and exterior durability.
Traditional RAL colours
We can produce any specified traditional RAL colour for any type or size of project, be it for raw steel substrate in an interior or a galvanized substrate for an exterior.
Powder coating at Joseph Ash Galvanizing
Our dedicated powder coating facility at Joseph Ash Medway is the ideal location for powder coating your steel. Based in Kent, our site offers a collection and delivery service to the surrounding area, and offers a one-stop-shot of hot dip galvanizing, steel and aluminium powder coating, and shot blasting.
We can produce any specified colour or texture for any size or type of project you have, whether it’s a raw steel substrate in an interior or a galvanized substrate for an exterior.
Joseph Ash Medway is among only a handful of companies to be an approved AkzoNobel paint applicator, and is the only one to be based in the South East.
If you’ve decided to have your steel hot dip galvanized to protect it from corrosion, you may be wondering if you need to do anything before you send it to be coated.
Whilst your galvanizer will take care of the galvanizing process for you, there are some things you must do before the galvanizer collects your steel. In this blog, we cover some pre-galvanizing preparation steps before you send your steel off to ensure a safe, quick, and effective process.

Design right for galvanizing
An important requirement for hot dip galvanizing is to make sure the steel has been correctly designed for the process. This includes adding ventilation holes into your steel, to make sure the molten zinc can flow freely over all internal and external surfaces and can fully drain. Otherwise, trapped air or process chemicals could cause a reaction, leading to zinc explosion. We have created a series of YouTube videos which detail what can happen if steel is not designed for galvanizing, including examples with various fabrication types
Correct design is important for keep our operatives safe and ensuring your steel is returned to you without damage. Fortunately, we’re here to help you make sure your steel is right before galvanizing. The Safe Design for Hot Dip Galvanizing flyer can be downloaded to help you better understand the technical requirements when it comes to creating vent holes in your steel. Alternatively, contact your galvanizer if you need any more assistance with venting.
Check the bath dimensions
The steel will also need to be able to fit into the galvanizing bath. This should not be a problem for many fabrications; however, it is important to check the bath sizes and dipping capabilities of your galvanizer to know what can and can’t be dipped. Double dipping is usually possible, meaning that sizes larger than the bath dimensions could be coated.
If you’re struggling for suitable locations, Joseph Ash Galvanizing is home to the widest (Telford) and deepest (Bilston) baths in the UK, suitable for dipping your large steel fabrications. Joseph Ash Chesterfield is also among one of the longest baths at over 16 metres.
For smaller steel pieces, a process known as spin galvanizing will need to be used. It follows the same fundamental process at hot dip galvanizing, except the steel will need to be placed into a perforated basket first to submerge it in the bath without losing any pieces. It is then “centrifuged”, or spun at high speeds to remove excess zinc.
Not every galvanizer caters for this process, so it is important to research into spin galvanizing providers if you need to coat small pieces such as nuts, bolts, fasteners and fixings. Joseph Ash Telford has a specialist spin galvanizing facility alongside a standard hot dip galvanizing bath.
Make sure it is clean
Ensuring the steel is free of any dirt or contaminants before it is galvanized helps the zinc adhere to the underlying steel. This helps to make the galvanizing process as effective as possible.
Contaminants could also cause a reaction with the zinc in the galvanizing bath, causing explosion from the chemicals mixing together. Articles should be free from oil, grease, paint, welding slag, lacquer, labels and glue etc. when sent for galvanizing. Shot blasting is a method of removing rust and unwanted layers from steel, helping to make the galvanizing process more effective.
Your galvanizer should check over the steel’s surface before it goes into the bath, but doing so beforehand helps reduce the amount of time the process takes. If your steel is particularly dirty, your galvanizer could refuse to accept it.
Load it safely
At Joseph Ash Galvanizing we offer a collection and delivery service, helping to save our customers time. But if you’re arranging your own transport, or are otherwise in any way involved in the delivery process, it is important to ensure you know how to stack, pack, load and secure your products for transporting them safely.
We have created our own Safe Deliveries flyer which helps customers, drivers, and our colleagues understand the importance of correct health and safety measures when delivering to and from any site.
If in doubt, ask
It’s better to ask if you’re unsure of what to do before galvanizing. If you have any questions before sending your work for galvanizing, contact your local galvanizer today.
- We’ll collect and deliver your steel to our site ✔️
- Comprehensive technical support, guidance and assistance at every stage ✔️
- Hot dip galvanized to BS EN ISO 1461 standards ✔️
- Part of a circular economy ✔️
What is the best steel coating method?
Hot dip galvanizing and commercial paint are two popular steel coating methods, used to help protect steel from corrosion and extend its lifespan. However, you may be wondering whether galvanizing or paint is better for protecting your steel.
We compare galvanizing and paint as coating methods for steel, on a number of different metrics to help you decide which is better for your steel fabrication.

Round 1: Performance
When it comes to performance, hot dip galvanizing can last for several decade maintenance-free under usual circumstances. In the right environment, galvanized steel can last over 100 years against corrosion.
In comparison, paint on steel lasts for a maximum of around 20 to 25 years. While this is still quite a long lifespan, this is only if the steel is kept away from almost all external variables, such as bad weather.
When comparing galvanizing and paint like-for-like on performance, galvanizing is the clear winner, having a much longer lifespan in different types of environment.
Round 2: Coating adherence
Adherence is especially important when it comes to coating your steel, because if it the coating can’t stay on the steel, it no longer protects the steel from corrosion.
Paint can stay on steel for a while, assuming the steel doesn’t get knocked or handled too much. However, because paint is a surface-level coating, it can easily chip off, leaving the underlying steel exposed.
Hot dip galvanizing, on the other hand, creates a metallurgical bond with the steel. This is because of the reaction that happens when the molten zinc from the galvanizing bath makes contacts the steel. The reaction makes the zinc coating becomes part of the steel itself, so it doesn’t chip away. The coating is much more durable as a result, which is what gives steel an enhanced lifespan.

Round 3: Resistance to damage
Depending on where your steel is erected, or what it’s being used for, it’s likely that it will be handled or impacted a lot. This can wear away the coating, leaving your steel vulnerable to corrosion.
We’ve already covered that molten zinc forms a metallurgical bond with the underlying steel, which is how the coating lasts for so long. This bond means that it is also very resistant to mechanical damage, so you can handle your steel without worrying about removing its corrosion-resistant properties.
Because paint only provides a simple bond to the steel, it means it is more prone to mechanical damage. Additional care needs to be taken when handling to ensure the coating does not get damaged. This makes paint unsuitable for steel that needs to be handled a lot, such as large structural steel fabrications or steel that is used in transport.
Round 4: Weather resistance
Most steel comes into contact with the outside world, which means it needs to be resistant to rain, snow, and hail, as well as humid and fluctuating temperature environments.
The longevity of paint coatings is entirely dependent on the environment the steel is in. If it is too hot or humid outside, the painted coating is compromised and could peel off of the steel. This leaves the underlying steel vulnerable to corrosion – especially from rain – as it can get into the cracks left in the coating.
Galvanized steel doesn’t become compromised until at least 200°C (390°F) – far hotter than the hottest day on record! Galvanized steel is also resistant to corrosion from moisture, so even if it’s raining or it’s humid outside, the steel won’t corrode easily.

Round 5: Coverage
In order for your steel coating method of choice to be effective, it needs to cover all areas of your steel. Otherwise, corrosion can develop in the uncoated areas, compromising the rest of the steel.
Painting steel is a very time-consuming and manual process. Because it is done by hand, there is lots of room for human error, making it easy to miss narrow corners and edges. Paint also only provides a surface-level coating. As mentioned previously, this makes it more susceptible to chipping and mechanical damage, leaving your steel vulnerable to corrosion.
Hot dip galvanizing, on the other hand, provides both internal and external coverage for steel thanks to the metallurgical bond that forms from the molten zinc. This makes galvanizing superior at protecting against corrosion by providing a consistent and reliable finish.
Galvanizing industry standards such as BS EN ISO 1461 also mean your steel will be galvanized to a specified coating thickness, further ensuring a reliable coating process.
Round 6: Whole life-cycle cost
When you compare the price of individual coating sessions, paint is cheaper than galvanizing. However, when it comes to the total cost of coatings over the course of the steel’s lifespan, galvanizing works out far cheaper. Paint doesn’t last as long, meaning you will need to frequently pay and spend time to recoat the steel whenever it needs amending.
According to the Galvanizer’s Association, a “cheaper” paint system is almost 70% more than the cost of galvanizing over the course of a 25-year project life.
Not only do you save money over time, but you can be sure your steel will last even longer against corrosion – a win-win.
And the winner is…
Galvanizing is superior to paint for performance, coating adherence, damage resistance, weather resistance, reliability, and whole-life cycle costs.
Contact your nearest galvanizing plant today for a quote on galvanizing your steel fabrications.
If your steel structures corrode, it’s not just financial costs you need to be aware of. It can also cost the environment and the health and safety of individuals.
We explore the costs associated with rusted steel on a personal and global level, and what can be done to mitigate these costs.

How much does rust cost to fix?
If left untreated, steel will inevitably corrode from elements such as oxygen, moisture, or chemicals. However, the speed at which steel corrodes can heavily impact global financial resources.
It is estimated that, globally, one tonne of steel will turn to rust every 90 seconds. Following a steady rate, that’s over 350,000 tonnes of steel in a year. This is a huge amount of steel to lose; it is estimated that corroded steel accounts for nearly 4% of Gross Domestic Product (GDP) lost every year.
From a global perspective, this has huge repercussions across many different sectors. Resources need to be used to manage, repair, or replace corroded infrastructure and equipment. This is a substantial financial burden, diverting funds away from other areas.
Costs to businesses
There are direct and indirect costs associated with steel corrosion for both individuals and businesses. The most immediate financial impact of corrosion is the cost of repairing or replacing damaged equipment and structures. This includes the cost of materials, maintenance and repair, and production downtime.
Indirect costs may include the inefficient running of machinery and other equipment due to corrosion. This can lead to higher energy consumption and increased operational costs.

Environmental cost of rust
It’s not just financial costs that are created from rusting steel – corroded steel can also harm the environment. CO² is emitted from corroded steel, with estimates of 1.6-3.2% of global CO² emissions being attributed directly to corroded steel.
If steel is particularly rusty, it may not be salvageable and safe to reuse. A new structure would need to be fabricated to replace it, but the steel fabrication process can be harmful to the environment. It has been estimated that new steel production accounts for 11% of all CO² emissions worldwide, contributing to climate change. Additionally, new steel fabrication requires raw materials, contributing to resource depletion and environmental degradation from mining and manufacturing processes.
Operational costs and safety risks
Machinery used in businesses across all kinds of sectors may become corroded, causing downtime, or slowed production lines. Downtime can also be extended to the unexpected failures of steel infrastructure, causing sudden downtime that can may impact finances and customer trust.
Health and safety can also be seriously compromised by corrosion; this may be especially true with load bearing steel infrastructure such as bridges and buildings. The failure of these fabrications can lead to catastrophic failures, endangering lives and causing serious injuries.
Save costs with hot dip galvanizing
There are many different costs associated with the corrosion process, but these can all be reduced by using a corrosion-resistant steel coating.
Hot dip galvanizing is a steel protection service that extends the lifespan of steel, allowing it to remain maintenance-free for multiple decades. In some circumstances, galvanized steel can last for over a century. Its extended lifespan for steel reduces financial impact by reducing the need for maintenance, refabrication, and recoating.
It’s not just financial costs you can save with hot dip galvanizing. It’s also a sustainable choice for coating steel, reducing environmental impact from logistics, refabrication, and emissions from corroded steel.
When you choose Joseph Ash Galvanizing, you can be sure you’ll receive a high quality finish from our friendly team that understands your unique requirements.
- We’ll collect and deliver your steel to our site ✔️
- Comprehensive technical support, guidance and assistance at every stage ✔️
- Fast turnaround times ✔️
- Hot dip galvanized to BS EN ISO 1461 standards ✔️
- Part of a circular economy ✔️
Cathodic protection helps prevent corrosion on a metal surface. We explain in detail what cathodic protection is, its different types, and the benefits of cathodic protection for steel, especially in the context of hot dip galvanizing.

Cathodic protection explained
Each type of metal has an different electric potential, or the amount of potential electricity it can hold. When two different types of metal come together, provided they have dissimilar potential, the metal with more electro-negative potential will “sacrifice” itself and corrode before the metal with less electro-negative potential.
There are two types of cathodic protection. One is achieved by supplying a direct current (DC) from an external source, known as impressed current cathodic protection. The other makes use of sacrificial anodes, known as galvanic anode or sacrificial anode cathodic protection.
Benefits of cathodic protection
By preventing corrosion, cathodic protection extends the lifespan of metal structures, reducing the amount of replacements or repairs needed. This helps to save time and money on refabrication.
The extended lifespan given to the steel also means that it becomes a more sustainable solution than steel without cathodic protection, by reducing emissions associated with transport and refabrication.
Cathodic protection also helps to make vital steel fabrications such as structural steel more safe to use. Corrosion poses a significant threat to steel which can, in turn, create serious safety hazards. The extended lifespan given by cathodic protection helps to ensure that the steel remains reliably safer to use for longer and saves on downtime associated with maintenance.
Galvanizing provides cathodic protection
The hot dip galvanizing process provides galvanic anode cathodic protection for steel. The fabrication is dipped into a bath of molten zinc, causing a reaction that makes the zinc bond to the underlying steel. This occurs because zinc has more electro-negative potential than iron, causing the zinc to corrode first.
The cathodic protection provided through galvanizing means that it is a much more effective and long-lasting coating method than alternatives such as paint.

A high quality coating
Although hot dip galvanizing is very effective at corrosion protection, it’s important to choose the right galvanizing company for the job.
A bad quality job may result in an uneven coating thickness, which can cause a quicker rate of corrosion than a coating with the same thickness throughout. Spikes on an uneven coating could also cause a potential safety hazard. An uneven coating also may not look as nice, which may be important to you depending on its use. A high-quality galvanizing company will ensure the galvanized finish looks smooth, shiny, and aesthetically appealing.
When you choose Joseph Ash Galvanizing, you will be working with a company that cares about quality and takes care of the process from start to finish.
- We’ll collect and deliver your steel to our site ✔️
- Comprehensive technical support, guidance and assistance at every stage ✔️
- Fast turnaround times ✔️
- Hot dip galvanized to BS EN ISO 1461 standards ✔️
- Part of a circular economy ✔️
Can galvanized steel survive extreme temperatures?
Galvanizing is a popular method of protecting steel against corrosion. But is galvanized steel resistant to extreme temperature environments, such as extreme heat or cold? Read on to learn about what temperature environments galvanized steel is suitable for.

Galvanized steel in high temperatures
Galvanizing is a long-lasting method of protecting steel against corrosion. It involves dipping steel into a bath of molten zinc, which creates a metallurgical reaction that protects the underlying metal.
The steel industry has generally recommended the service temperature for conventional coatings to be less than 200°C (390°F).
200°C is very high – much hotter than most environments – so it is unlikely that your steel fabrications will be affected by heat.
It is important to be aware that at temperatures above 200°C (390°F), you may experience peeling and changes in mechanical properties.
What is peeling?
Peeling is caused by metallurgical changes in the steel, creating a series of closely-spaced “voids” at the free zinc-alloy interface. They are produced from the diffusion of zinc from the outer free zinc layer into the inner alloy layer, caused by a difference in diffusion rates between them.
These voids expand and form a gap, causing the outer free zinc layer to split-off from the underlying zinc-iron alloy layers. This process is known as the Kirkendall Effect.
However, the remaining zinc-iron alloy layers will still provide corrosion protection for many years. How long these protective qualities last depends on the remaining coating’s thickness. At temperatures between 200°C (390°F) and 250°C (480°F) the zinc-iron alloy layers will continue to protect the steel from corrosion.
Temperatures above 250°C (480°F) will speed up the peeling process, and continued exposure can result in the zinc-iron alloy layers cracking and separating from the steel. Figure 1 shows the peeling of galvanized steel versus the temperature of the environment. For temperatures of 200°C or below, the coating resists zinc layer peeling, making hot dip galvanizing a suitable choice for these circumstances. Brief temperatures exposure up to 300°C may result in no damage to the coating.
Variables that influence steel deterioration rate
Apart from time and temperature, the rate of the deterioration process is influenced by coating thickness, the relative thickness of outer zinc and zinc-iron alloy, and by the uniformity of the individual layers. Any of the coating’s factors can affect the speed and degree of deterioration because of the effect on the length of the zinc diffusion path, or the rate of the iron-zinc interdiffusion reaction.
Peeling occurs at temperatures above 200°C, and the amount is dependent on the rise in temperature and the duration exposed. However, peeling does not mean corrosion protection is lost. During peeling, only the outer free zinc layer becomes detached, leaving the zinc-iron alloy layers to provide corrosion protection to the steel.
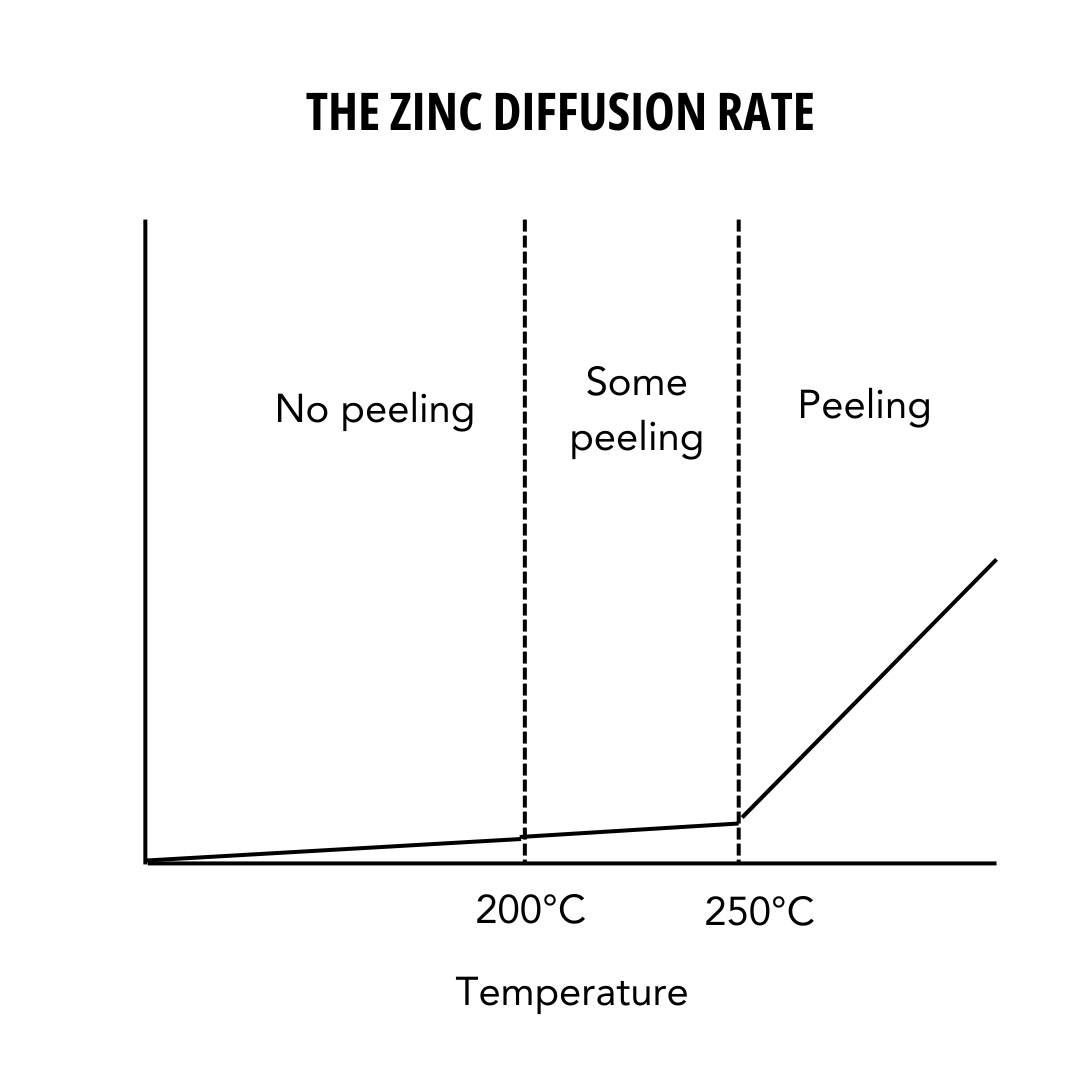
Figure 1. The Zinc Diffusion Rate
Other variables that can impact steel’s deterioration rate include moisture levels and contact with chemicals, such as pesticides.
Is galvanized steel fireproof?
Temperatures in fires can easily exceed 600°C. As galvanized steel starts to peel at above 200°C, this means that long-term exposure to fire can cause the steel to deteriorate. However, in short bursts, there is a potential for coating damage but many have found fire damage to be minimal on galvanized steel. A layer of carbon dust often coats the galvanized surface, but under this layer, the coating stays intact.
Changing mechanical properties
When heating at 400°C, exposures from two weeks up to 16 weeks produce relatively minor changes to the mechanical properties in the steel. The steel’s yield strength – the maximum stress that can be applied before deforming – is not significantly altered, but there is a decrease in tensile strength. These structural changes are insignificant and do not affect the design of steel structures.
Galvanized steel in cold temperatures
Unlike extreme heat, extreme cold appears to cause minimal change in galvanized coatings.
Sources have suggested that galvanized steel is unaffected in temperatures at -40°C (-40°F). Therefore, low temperature climates are an appropriate environment for hot dip galvanized steel.
However, as with any steel at very low temperatures, the material becomes brittle with extended use.
Takeaway
The recommended service temperature of 200°C has been a good benchmark for coating protection on galvanized steel with no free zinc peeling, meaning galvanized steel is suitable for most environments.
At temperatures ranging between 200°C (390°F) and 250°C (480°F) the zinc-iron alloy layers will continue to protect the steel from corrosion, but may result in some peeling. Brief temperatures exposure up to 300°C may result in no damage to the coating, but is not recommended for long-term exposure.
Colder service temperatures cause minimal change to the coating, meaning galvanized steel is ideal for these environments.
Galvanizing services in the UK
No matter your sector or size, we’re here for your galvanizing requirements. We have nine UK sites who are ready to hear from you.
- We’ll collect and deliver your steel to our site ✔️
- Comprehensive technical support, guidance and assistance at every stage ✔️
- Hot dip galvanized to BS EN ISO 1461 standards ✔️
- Part of a circular economy ✔️
Galvanized steel can last a lifetime, so it’s expected that it won’t stay looking like new forever. Dirt can build up from everyday mud and grime, as well as from industrial sources. Therefore, you may be wondering how to clean galvanized steel.
While you can clean steel before galvanizing it with shot blasting, the galvanized finish on the steel will be compromised if you try to shot blast it after galvanizing. Fortunately, there are ways of cleaning galvanized steel that don’t damage the coating.

Cleaning galvanized steel
There are several ways of treating stains or marks on galvanized steel. Cleaning should be conservative at first, becoming more vigorous as needed. This is to ensure you don’t damage the zinc coating.
When cleaning bulk contaminants such as dirt, ordinary soaps can be used. For more adherent contamination or for larger areas, the use of a low-pressure wash with pure water, or alongside proprietary cleaning materials such as car wash fluid, can be effective. The car and truck cleaners are formulated to minimise corrosion on the metallic parts of vehicles so are suitable for use on galvanized steel. However, it is important wash the steel with fresh water afterwards.
Many mild stains (such as those from water ponding and water runs) can be removed with the use of common household ammonia cleansers. Again, be sure to thoroughly rinse the galvanized article with fresh water afterwards.
Finally, do not use any abrasive materials on the steel, as this can wear away the zinc coating achieved from the galvanizing process. It’s best to use a water-based emulsifier; alkaline-based cleaners with a pH of 12 or lower; or organic solvents for cleaning galvanized steel.
After cleaning, rinse the area with clean water and wipe the steel with a soft cloth. Make sure that you fully dry the steel to avoid the formation of wet storage stain on the surface.
Removing cement from steel
During building or renovations, cement and mortar may be dropped onto the galvanized steel. This can be very difficult to remove from the steel once it has hardened. If this occurs, remove the large parts of the deposit as close to the surface as practicable. Oxalic acid can then be used to remove the remaining remnants from the galvanized steel, followed with a thorough rinsing. Other acids are more effective on the mortar or cement, but these can be very damaging on zinc and are not recommended.
How to remove wet storage stain
Wet storage stains, also known as white rust, are common when galvanized surfaces are exposed to moisture (such as rain, dew, or condensation) and there is limited air flow over the surface. Wet storage stains can be prevented by storing galvanized steel in ventilated areas away from moisture.
While it’s best to prevent white rust rather than remove it, you can clean it by using a diluted acidic or ammonia solution, followed by a thorough rinsing with clean water.

How to touch up galvanized steel
Over time, there may be some areas that lose their galvanized coating. This can happen if you clean your steel incorrectly, or through gradual damage that builds up from everyday movement and impact. Fortunately, patches of damaged steel can be touched up with a zinc spray.
Zinc sprays contain condensed amounts of zinc that you can spray onto affected areas, without having to re-galvanize the whole fabrication. This saves you time and money compared to re-galvanizing the whole fabrication. It also helps extend the lifespan of steel as it prevents rust from building up inside the damaged areas, which may save you from needing to rebuild the fabrication from scratch.
One brand of zinc spray you can use is Galvsafe Zinc Spray. Galvsafe Zinc Spray is safe to paint and powder coat over, making touching up your steel easy. The Zinc Spray also colour-matches steel, so it blends in seamlessly and keeps the fabrication looking like new. It’s ideal to use after cleaning your galvanized steel to help maintain its lifespan. Order yours today by contacting your nearest Joseph Ash Galvanizing site.
If you have any questions about cleaning your galvanized steel, get in touch with your local galvanizer for advice.
Ready to galvanize your steel?
Many industries rely on their steel fabrications being kept outside. From structural steel buildings to farm gates and transmission towers, steel is prone to corrosion in moist environments. Fortunately, hot dip galvanizing serves as the perfect coating method for steel that is exposed to the elements.
What happens when galvanized steel gets wet?
Normally, when steel gets wet – whether from the rain or moisture in the air – it corrodes very quickly. When metals collect moisture, an electrochemical reaction causes the steel to corrode. The corrosion process is often accelerated by dissolved salts or impurities in the water.
Rust is just one type of corrosion but it’s one of the most prevalent types to affect steel. It occurs when oxygen reacts with metal atoms, forming metal oxides. Both oxygen and moisture are required for steel to rust. However, it’s very unlikely your steel will be kept away from oxygen if it’s exposed to moisture. Therefore, it’s important to slow down the corrosion process as much as possible.
The galvanizing process involves dipping steel into a bath of molten zinc, causing the zinc particles to metallurgically bond to the steel. This bond creates a much more durable finish that surface-level coatings such as paint, meaning galvanizing is effective for protecting steel against atmospheric attack, such as heavy rain.
Is galvanized steel good for outdoor use?
Galvanized steel is good to use outdoors thanks to its corrosion-resistant properties. Structural steel, transmission poles, and agricultural equipment are all types of steel that are likely to be affected by weather conditions, among many other types of steel fabrications.
If you’re in the construction, agricultural, transport or energy sectors, galvanizing steel that is used outside means you save time and money on maintenance and replacements.

Outdoor paint for steel
Hot dip galvanizing is a reliable coating method for protecting steel against weather damage. However, you may be looking to add an extra layer over your steel such as paint.
Paint is often used to give colour to steel and add an extra layer of protection, but it can chip away easily, compromising the underlying steel. Even when using waterproof paint, it is advised that you recoat it every 5-10 years. But what if there was an alternative to paint that doesn’t need retouching so frequently?
Fortunately, there is. Powder coating is a method of coating steel that adds colour without compromising on durability.
Powder coating is the best alternative to paint. Unlike paint, which chips easily and does not protect against corrosion, powder coating is applied electrostatically to the steel. This application technique means that the layer of powder coating protects the underlying steel against corrosion. Powder coating used in synergy with hot dip galvanizing is known as a duplex coating.
Joseph Ash Galvanizing for durable steel
With nine UK sites, we’re never too far away to protect your steel.
- We’ll collect and deliver your steel to our site ✔️
- Comprehensive technical support, guidance and assistance at every stage ✔️
- Hot dip galvanized to BS EN ISO 1461 standards ✔️
- Part of a circular economy ✔️
Welding is a versatile and essential skill for steel fabricators. However, you may wonder if it is safe to weld steel after it has been galvanized. Does the zinc coating affect the welding process? Will the welding process ruin the galvanized coating? We explore whether you can weld galvanized steel, and the best practices to do so safely and effectively.

What is galvanized steel?
Prior to welding your galvanized steel, it’s crucial to understand the material you’re working with. Hot dip galvanizing involves dipping steel into a bath of molten zinc, creating galvanized steel. This zinc coating protects steel against corrosion, increasing its longevity and making it an ideal choice for fabricators.
Ungalvanized steel does not have this layer of zinc over it, which makes it more susceptible to corrosion. However, the zinc can cause problems when welding steel fabrications.
What happens when you weld galvanized steel?
When you expose galvanized steel to high temperatures during welding, the zinc coating evaporates, releasing toxic zinc oxide fumes and creating health and safety hazards.
Inhaling zinc oxide fumes can cause symptoms such as nausea, chills, fever, and muscle aches. These symptoms together are sometimes known as “metal fume fever”. Prolonged exposure to zinc fumes can cause serious health issues. Welding galvanized steel can also result in increased spatter and smoke, which can be hazardous and affect the quality of your work environment.
The quality of the welding job can also be affected. The presence of zinc during welding can create defects in the weld, such as porosity or inclusions, which weaken the joint.
Can you weld galvanized steel safely?
Although not recommended, you can still weld galvanized steel. However, it requires careful preparation and precautions to ensure your safety and the quality of the weld. Here are some steps to follow:
- Proper ventilation: Welding should always be done in a well-ventilated area, but it’s even more crucial when working with galvanized steel.
- Wear appropriate safety gear: Ensure you are wearing appropriate personal protective equipment (PPE), including a welding helmet, gloves, and respiratory protection.
- Clean the surface: Before welding, grind away the zinc coating from the areas you intend to weld. This prevents the release of zinc fumes and ensures better weld quality.
- Use low heat: Reduce heat input by using lower amperage and voltage settings on your welding equipment. This helps minimise the evaporation of zinc.

Should I weld steel before galvanizing?
You can avoid emitting zinc fumes if you weld steel before galvanizing, reducing the side effects associated with inhaling zinc fumes. However, this doesn’t mean there aren’t health and safety risks with galvanizing steel that has already been welded. Fabricators need to ensure that their steel is correctly vented before sending to their local hot dip galvanizing site. Incorrectly venting your steel can have explosive consequences.
It’s also important to ensure you weld your steel together correctly by leaving seam gaps. Without gaps, air becomes trapped in the weld joint, causing pressure between the two welded pieces. This pressure builds up until the fabrication forcible separates at high speed. To avoid this, leave seam gaps between the steel pieces so that air can escape and avoid pressure building.
If in doubt, you can download our venting tips and tricks guide here.
However, it is important to note that the welded section will no longer be corrosion resistant after welding as the zinc coating is removed, leaving uncoated steel sections. Therefore, the item will likely have to be re-galvanized after welding to ensure it is protected.
To conclude, you can weld galvanized steel, but it requires extra care and precautions due to the zinc coating created from galvanizing. Safety should always be a top priority when working with any welding project, and this is especially true when welding galvanized steel. Proper ventilation, protective gear, and surface preparation are key to ensuring you remain safe when welding steel.
Ask your local galvanizing company if you are unsure about welding or venting steel.

